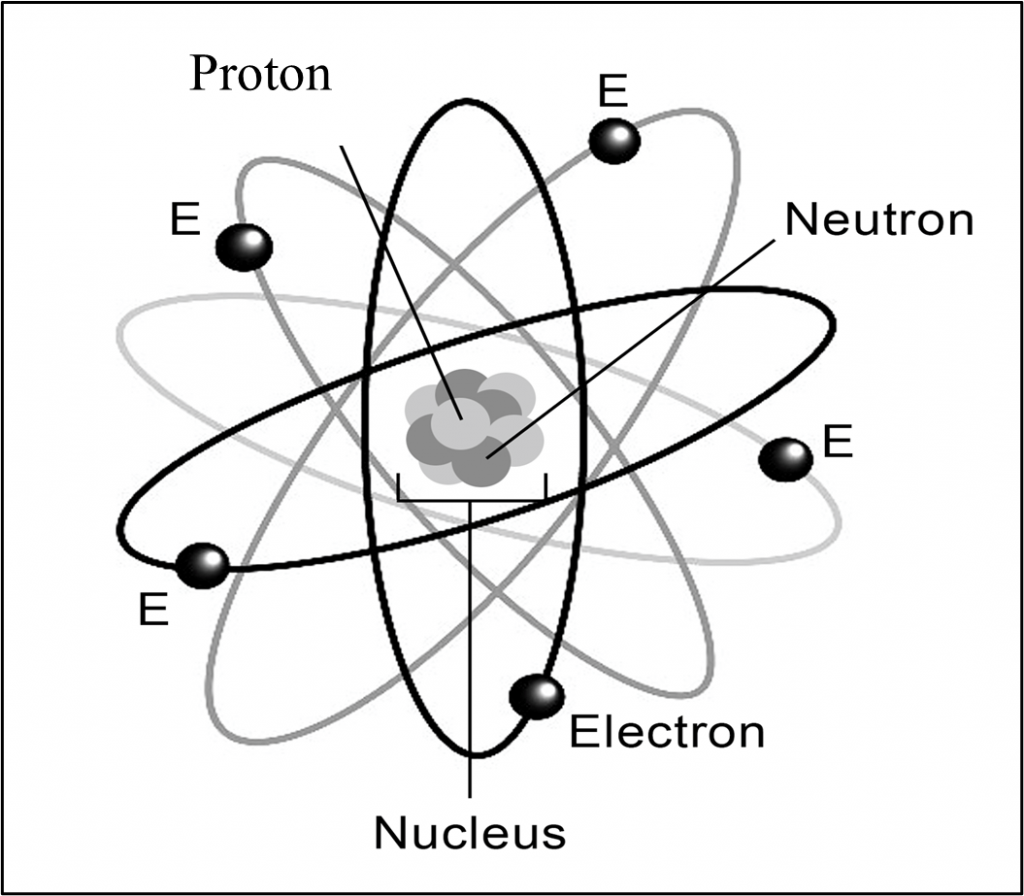
Atomic Structure
The smallest unit of an element is the atom. The atom has all the physical and chemical
properties necessary to identify it as a particular element. Atoms are composed of smaller particles including:
- Protons
- Neutrons
- Electrons
All living things are made of atoms, which structurally consist of a dense, central nucleus surrounded by a cloud of negatively charged electrons, a positively charged nucleus and one or more negative electrons. Protons and neutrons are heavy particles that are found in the center, or nucleus, of the atom. The basic difference between a proton and neutron is their associated electrical charge. Protons have a positive charge and neutrons have no charge. Electrons are even smaller, negatively charged particles that orbit the nucleus producing what is often described as a “shell” around the atom. Atoms contain the same number of electrons in orbit as they have protons in nucleus. This provides a neutral electrical charge overall to the atom.
The size of an atom is determined by the extent of electron’s orbits. If an atom could be enlarged such that the nucleus would be the size of a baseball, the outer electrons would be tiny specks nearly a mile away. To understand radon and its effect on the environment and human life one must first understand the basics of the atom and the concept of ionization and radioactivity.