Unstable Atoms
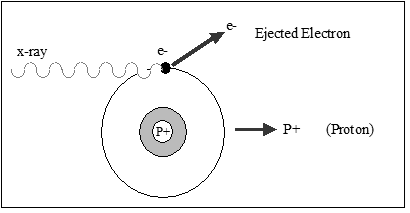
Everything in nature would prefer to be in a relaxed, or stable state. Unstable atoms undergo nuclear processes that cause them to become more stable. One such process called Ionization involves emitting excess energy from the nucleus, which physically changes an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. The resulting ion pair consists of a single electron and a single proton that are separated by a given distance. This process of emitting excess energy from the nucleus is called radioactivity or radioactive decay. The energy released from unstable (radioactive) atoms is called nuclear radiation.
This radioactivity, or Ionizing Radiation (Figure 2-3), consists of particles or electromagnetic waves energetic enough to detach electrons from atoms or molecules, thus ionizing them. The degree and nature of such ionization depends on the energy of the individual particles or waves, and not on their number. Exposure to radiation causes damage to living tissue and high doses can result in mutation, radiation sickness, cancer, and death.
| IONIZATION The process of converting an atom into an ion by gaining or losing electrons. Ionized atoms react with other matter to create radioactivity. Beginning stage of radiation. Creates a decay product of the parent isotope. |