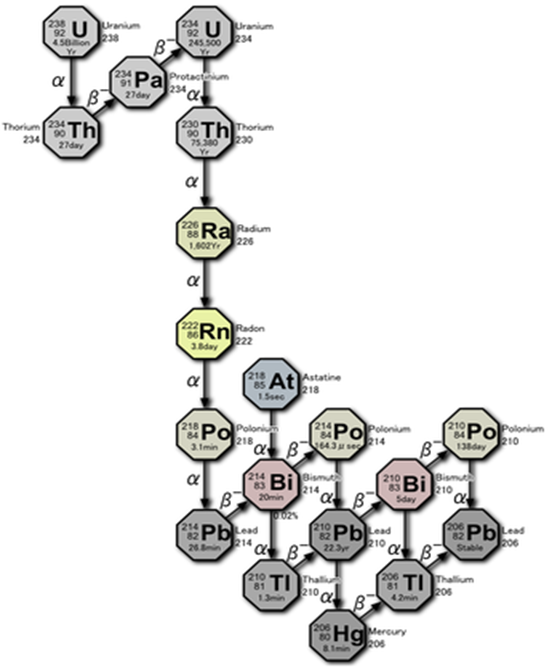
Uranium Decay Chain
Radon is part of the uranium decay chain. The decay of uranium produces radium after several intermediate steps. The numbers associated with the elements in Figure 2-7. are the atomic masses, which are the sum of the number of protons and neutrons in the nucleus of the atom. Most elements have many isotopes, each with their own half-life and decay products.
Remember, isotopes are different forms of an element that have the same number of protons but a different number of neutrons in their nucleus. For example, lead-214, lead-210 and lead-206 are all isotopes of lead. When referring to radioactive materials, we must be isotope-specific, since each element can have several isotopes of concern.
There are actually several different isotopes of radon. Currently, the primary isotope of radon that is of public health interest is radon-222, with 86 protons and 136 neutrons in its nucleus.